SAFE PRODUCTION OF WATER FOR INJECTION ACCORDING TO GMP AND FDA RULES
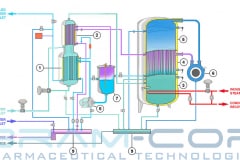
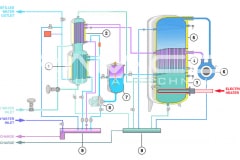
Water for Injection (WFI) can be produced through distillation or, also, through a downstream unit of ultrafiltration in a Reverse Osmosis system. The more safe methods are the distillation processes, such as vapor compression distillation or multiple effect distillation.

Therefore, Vapor compression distillation is a formidable distillation system because it uses a very profitable technology: the mechanical compression of the vapor generated by the machine, which involve a quick change of state.

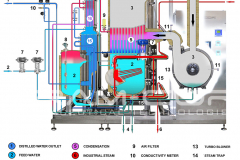
Not only that, but it is also necessary to consider the design and construction quality of the entire distillation system, suitable for use in the pharmaceutical industry. BRAM-COR project drivers are aimed at satisfying all pharmaceutical regulatory and QA requirements, aligning the Vapor Compression Distiller manufacturing to the international cGMP (Good Manufacturing Practices) and Pharmacopoeias.

Furthermore, there are customized models with pretreatment systems integrated on the same skid: STMC AEL/AST, with automatic water softeners, and REL/RST, with water pretreatment + reverse osmosis system. For this reason, BRAM-COR STMC vapor recompression still can virtually work with any water available on site.

For more info about Vapor Compression Distillation, see vapor-compression-distiller.com
Click here to see (main Bram-Cor site) all the pharmaceutical lines (turnkey, water treatment, processing, filling and packaging systems).
© Bram-Cor SPA 2024